Discoveries of Amerimmune Clinic in McLean VA, Alexandria VA, Arlington VA, Bethesda MD, and Gaithersburg MD
Amerimmune Clinic is at the forefront of medical innovation, with key discoveries including the use of caspase inhibitors for COVID-19 and RNA viruses, dupilumab as a potential treatment for FPIES, and basophils as biosensors for more accurate food allergy testing. These breakthroughs demonstrate our commitment to improving patient care through cutting-edge research. For more information, contact us or appointment online. We have convenient locations to serve you in McLean VA, Alexandria VA, Arlington VA, Bethesda MD, and Gaithersburg MD.


Discoveries of Amerimmune
Caspase inhibitors as host targeted therapy in COVID19 and RNA viruses:
The first description of elevated Caspase-1 levels and the role of pyroptosis in COVID19 patients. This finding has led to designing of a clinical trial to assess Caspase inhibition as a potential treatment modality in COVID19. The Phase 1 study showed safety and signals of efficacy in outpatient mild COVID19
Kroemer A, Khan K, Plassmeyer M, Alpan O, Haseeb MA, Gupta R, Fishbein TM. Inflammasome activation and pyroptosis in lymphopenic liver patients with COVID-19. J Hepatol. 2020 Jul 6: S0168-8278(20)30437-2.
Premeaux TA, Yeung ST, Bukhari Z, Bowler S, Alpan O, Gupta R, Ndhlovu LC. Emerging Insights on Caspases in COVID-19 Pathogenesis, Sequelae, and Directed Therapies. Front Immunol. 2022 Feb 21; 13:842740.
Plassmeyer M, Alpan O, Corley MJ, et. al. Caspases and therapeutic potential of caspase inhibitors in moderate-severe SARS-CoV-2 infection and long COVID. Allergy. 2022 Jan;77(1):118-129.
Discovery of a disease-modifying treatment for Food Protein–Induced Enterocolitis Syndrome (FPIES)
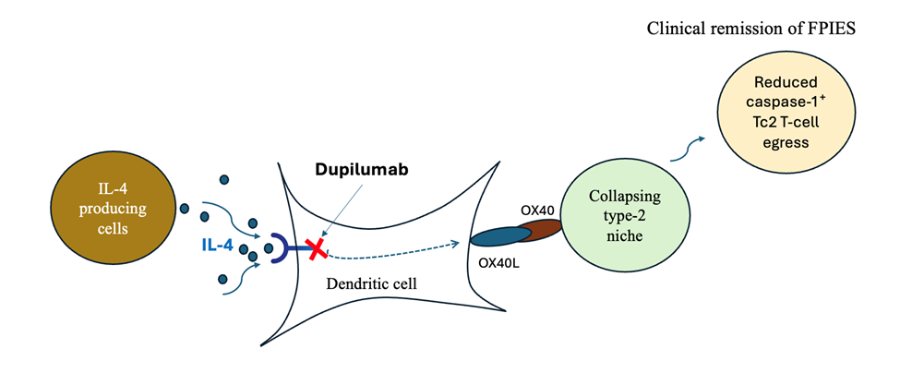
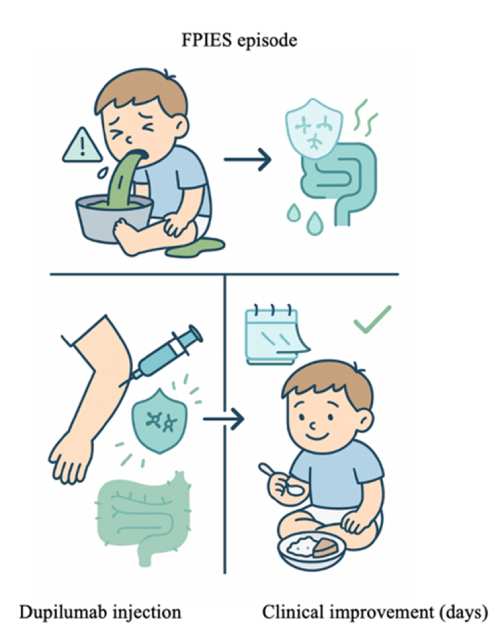
The FPIES discovery is that a pathway-targeted biologic, dupilumab (an IL-4Rα blocker approved for eczema, asthma, etc.), can unexpectedly switch off food-triggered FPIES-type gut reactions in a subset of patients.
It began with a single “experiment of nature”: a patient with lifelong, severe wheat-induced FPIES who, after starting dupilumab for eczema, accidentally ingested large amounts of wheat and had no reaction—then reliably relapsed when dupilumab was stopped and improved again when it was restarted. We then identified additional patients with FPIES or FPIES-like food-triggered enterocolitis who showed the same pattern: sustained tolerance to previously offending foods while on dupilumab, with recurrence of symptoms when the drug was withdrawn.
Together, these cases strongly suggest that at least one endotype of FPIES is driven by a type-2 cytokine/OX40L-linked pathway that is druggable with IL-4/IL-13 blockade, opening the door to the first mechanism-based therapy (and biomarker-guided stratification) for this historically untreatable, non-IgE food allergy.
Matthew Plassmeyer, Ph.D. Naomi Enav,Michael Girgis, Ph.D., Mikell Paige, Ph.D., Linda Todd, RNP, Laureana Israni, Oral Alpan, M.D. Dupilumab Opens a Therapeutic Window in Food Protein Induced Enterocolitis Syndrome by un-licensing dendritic cells
https://www.jaci-global.org/article/S2772-8293(25)00193-6/fulltext
Basophils as Biosensors:
The basophil activation test (BAT) functions as a living biosensor for allergens by using basophils from highly sensitized and reactive patients. We name this approach as In BAT, fresh whole blood is exposed in vitro to candidate allergens—foods, drugs, or formulas—and basophil up-regulation of activation markers such as CD63 is quantified by flow cytometry. Because the readout is a functional response (degranulation/activation) instead of just the presence of IgE, BAT can distinguish clinically relevant allergens. This makes BAT an ideal biosensor platform to compare unknown or engineered products (e.g., hypoallergenic formulas, new food ingredients) against known controls, detect trace allergen contamination, and, in our work, provide a regulatory-grade in vitro alternative or adjunct to risky oral challenges.
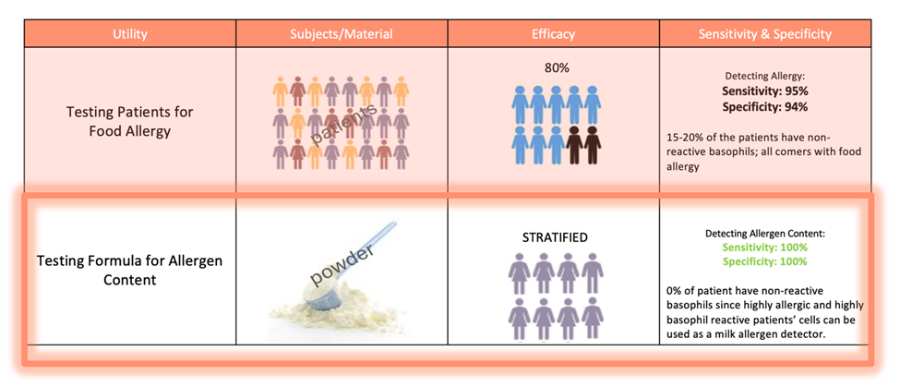

We are working with one of our partners to help reshape the U.S. regulatory pathway for hypoallergenic infant formulas, using this process to support hypoallergenic labeling claims.
Capabilities
• All phases of clinical trials
• No CRO support needed for the proof-of-concept studies
• Research and CLIA/CAP lab to support all functional biomarkers studies
• Discovery
• Intellectual Property
• Blood draw capabilities across USA (Quest)
• Robust Patient Recruitment resources
• Active Clinical Immunology Clinic
Collaborations:
• George Mason Chemistry department
• NY Allergy and Immunology Research Lab
• MetroImmune
Intellectual Property (published cases)
Postural Orthostatic Tachycardia Syndrome and CRTH2. Application #: 17/440620. Filing date: 9/17/2021
Treatment for Diseases Caused by RNA Viruses. Application #: 17/919020. Filing date: 10/14/2022
Treatment for diseases caused by RNA virus SARS-CoV-2. US12377127B2. Active. Expiration: 04-14-2041
Time Dependent Response of Basophils to Allergens, Application #: 18/213,417. Filing date: 23 Jun 2023


